TÜV SÜD @TUVSUD emite el primer certificado con el reglamento IVDR a un producto clase B – Enhorabuena!! | Red de Tecnologías Sanitarias y Productos Sanitarios

In Practice Series - How To Prepare For The European Medical Devices and In Vitro Diagnostic Regulations - Compliance & Risks

New European In Vitro Diagnostic Medical Devices Regulation - BDR | Blackhills Diagnostics Resources

CDSCO Recently Classified 80 In-Vitro Diagnostic Medical Devices For Regulation And Patient Safety - CliniExperts -CliniExperts

Publicado Manual sobre frontera y clasificación de productos sanitarios bajo MDR e IVDR ver.1 (sept 2022)

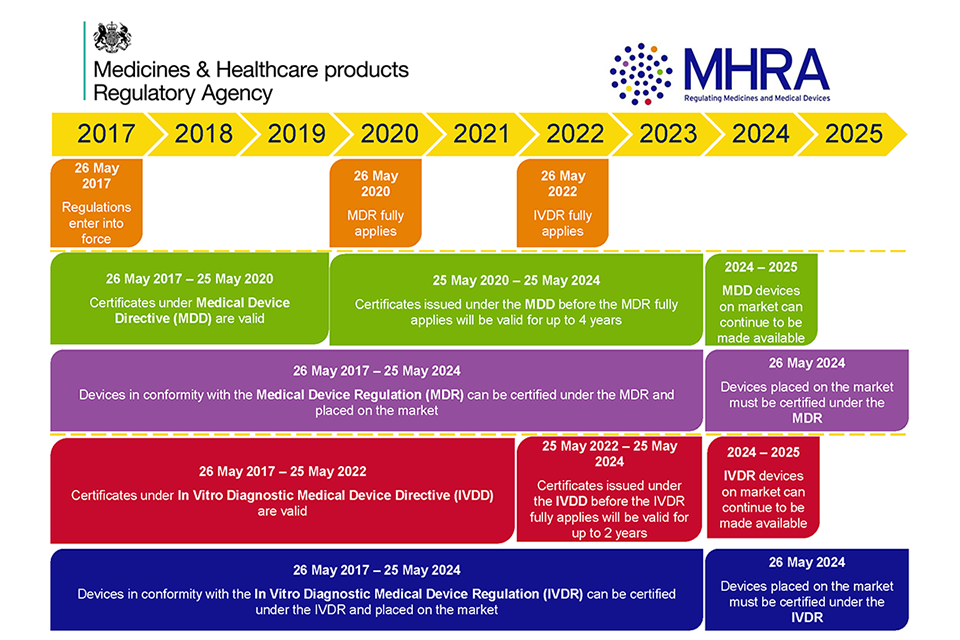







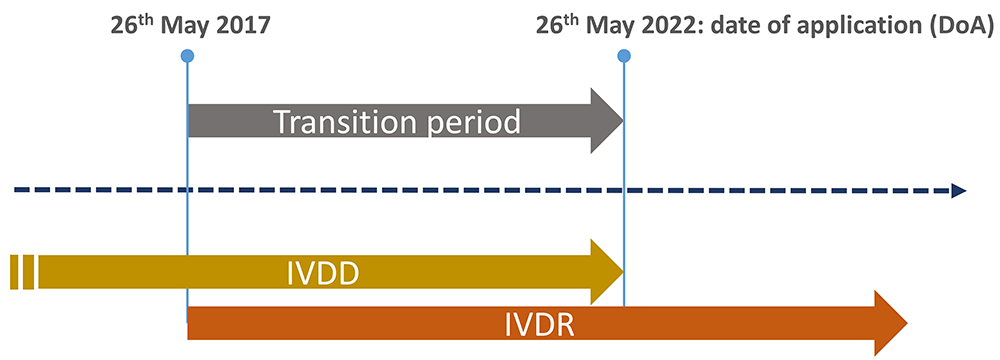



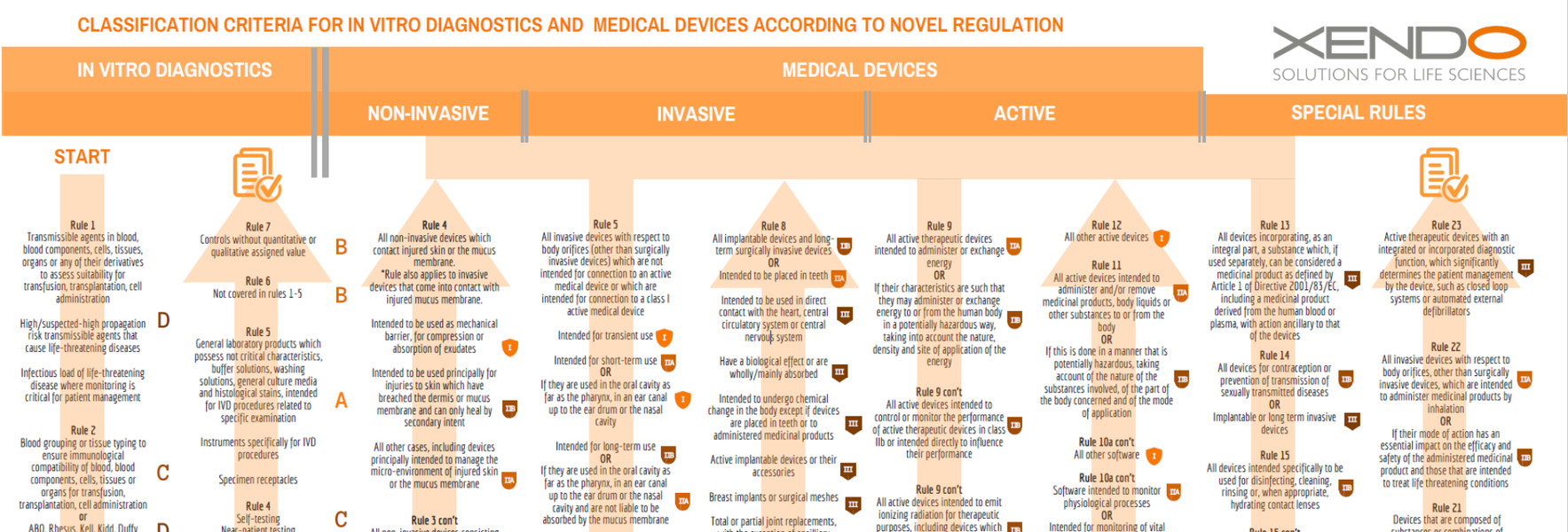
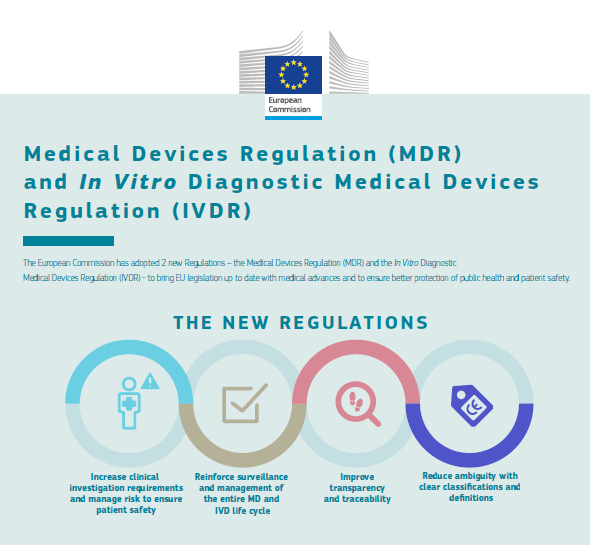
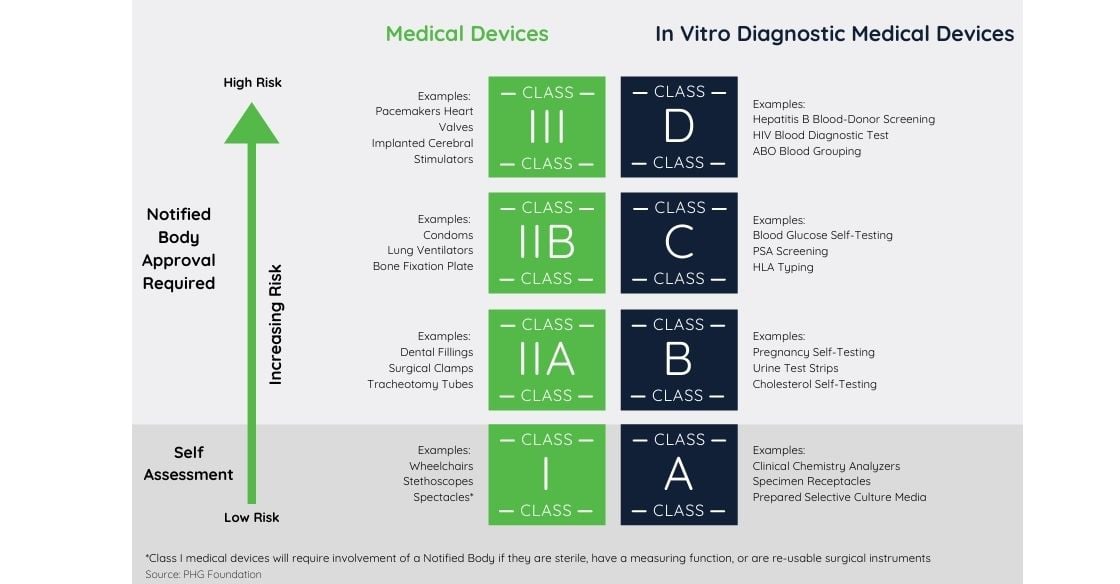
/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)