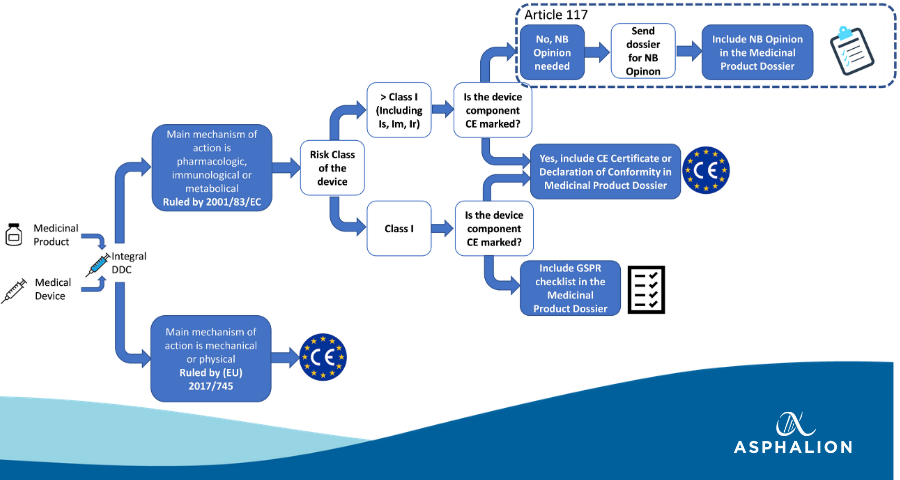
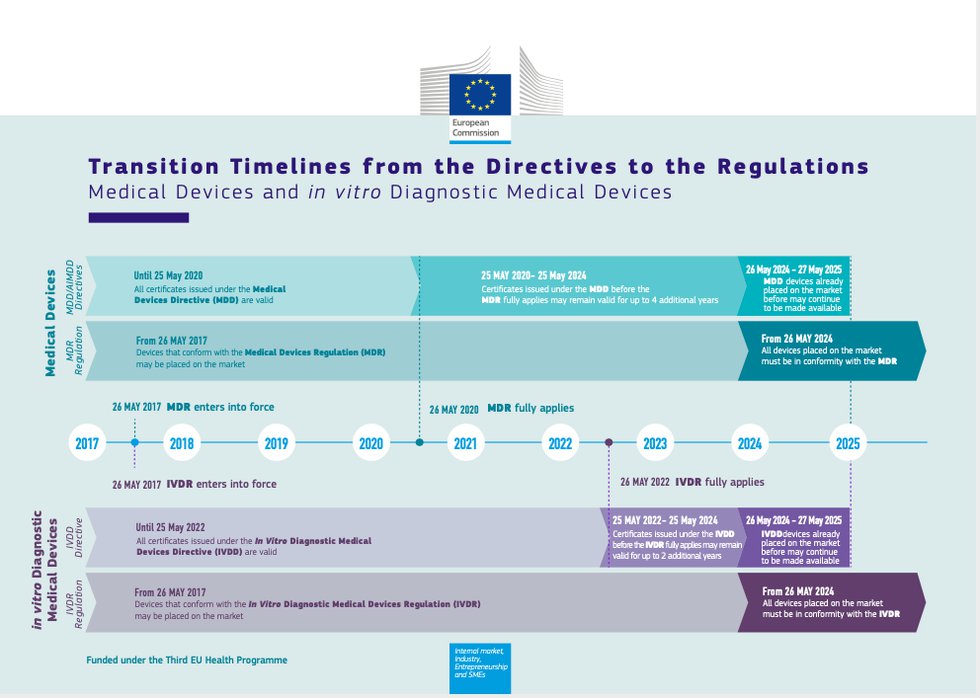
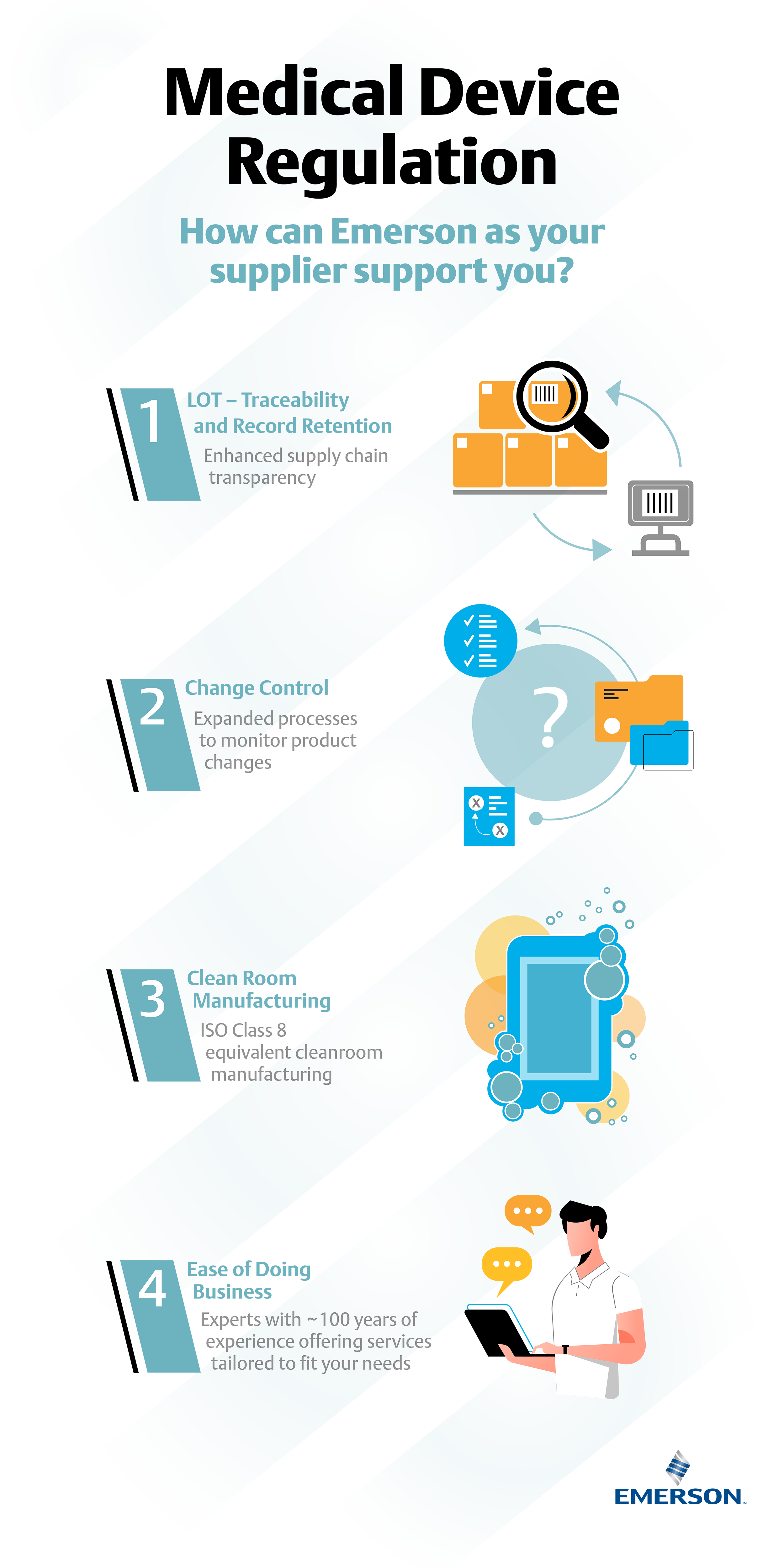
MDCG: nueva MDCG 2021-25 Aplicación de los requisitos de MDR a los productos 'legacy' y a los productos 'old'

Comply Guru - The European Medical Device Regulation (EU-MDR 2017/745) has come into effect today (May 26th 2021) and it will have an enormous impact on the #medicaldevices Industry If you need

Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention

Arion products are compliant with the new Medical Device Regulation 2021! • Bathing wipes gloves and compression stocking aids supplier