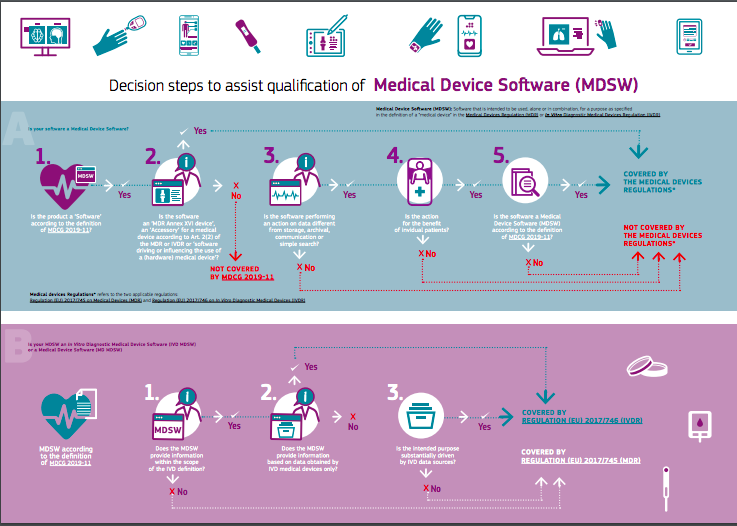
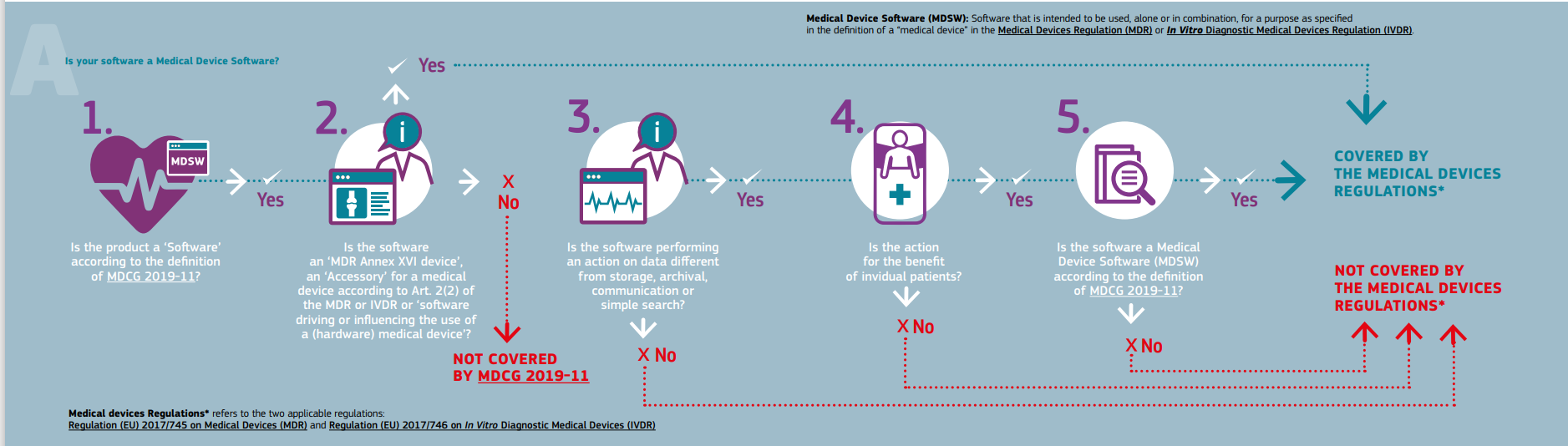
Medical device stand-alone software including apps (including IVDMDs) by The British Healthcare Trades Association - Issuu
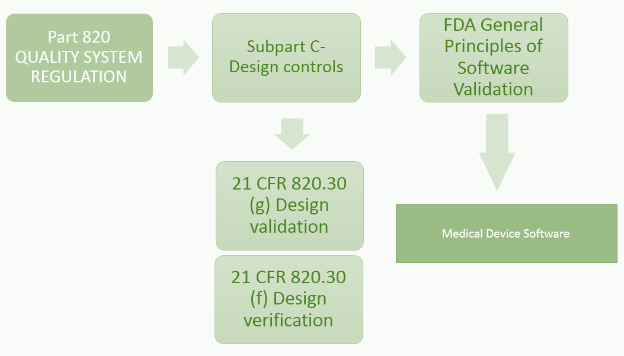
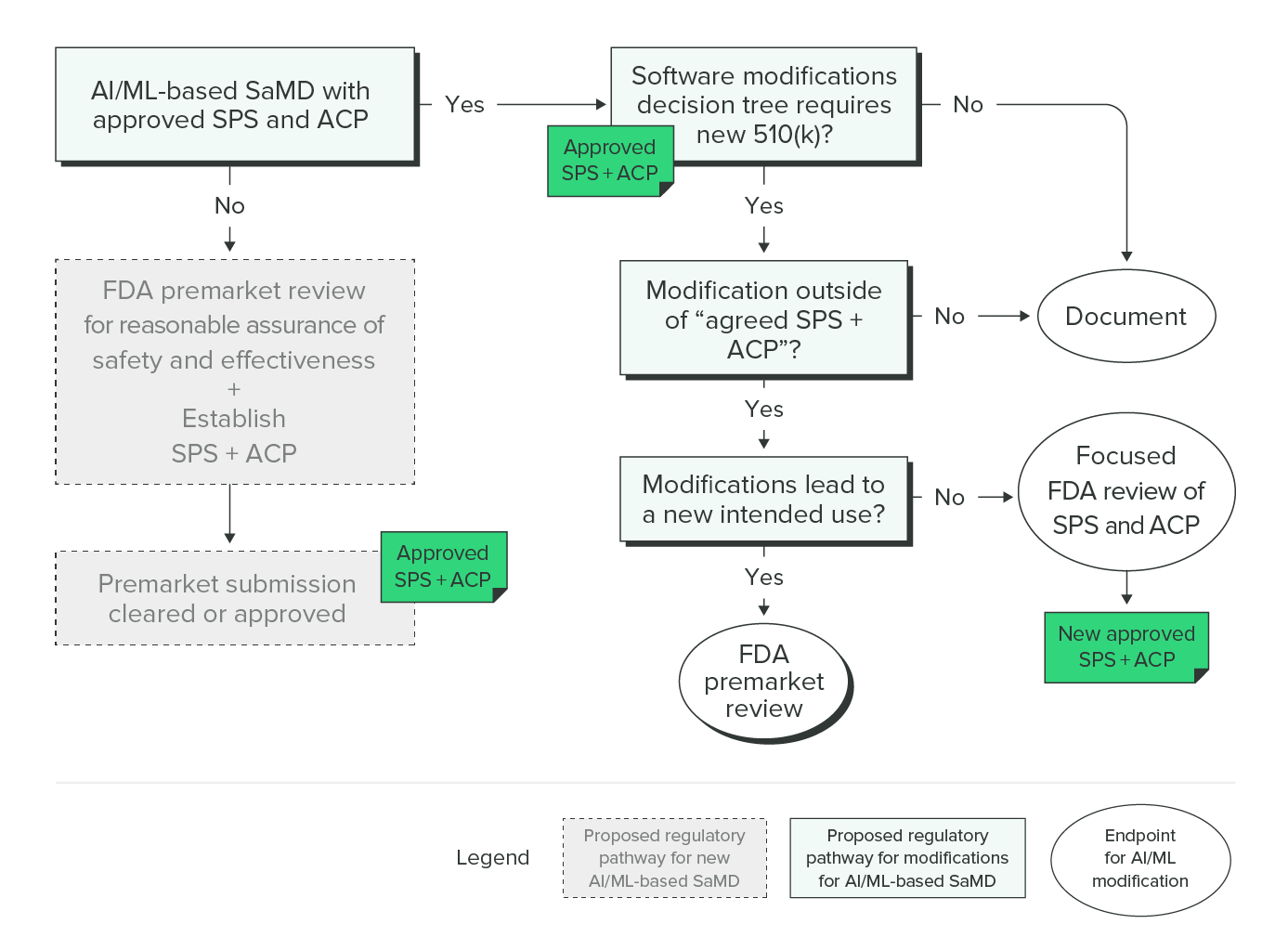
Addressing the Medical Device Software Challenges by understanding FDA's Software Regulation Strategy

On The Road to Medical Device Software Development | ICterra Information and Communication Technologies
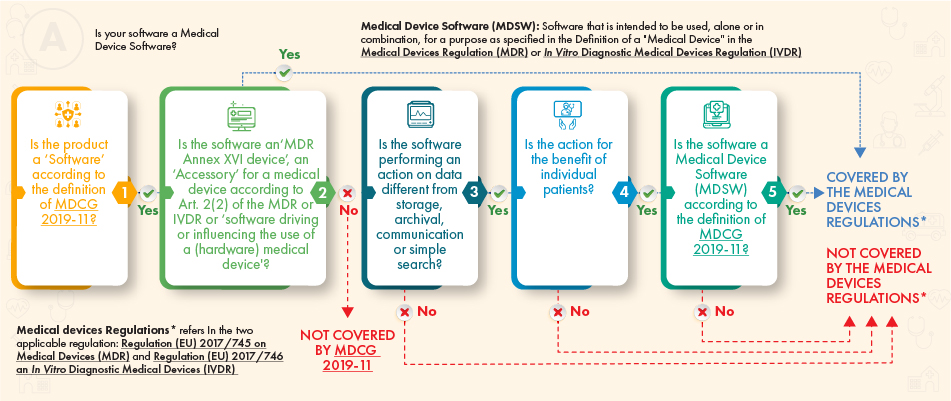
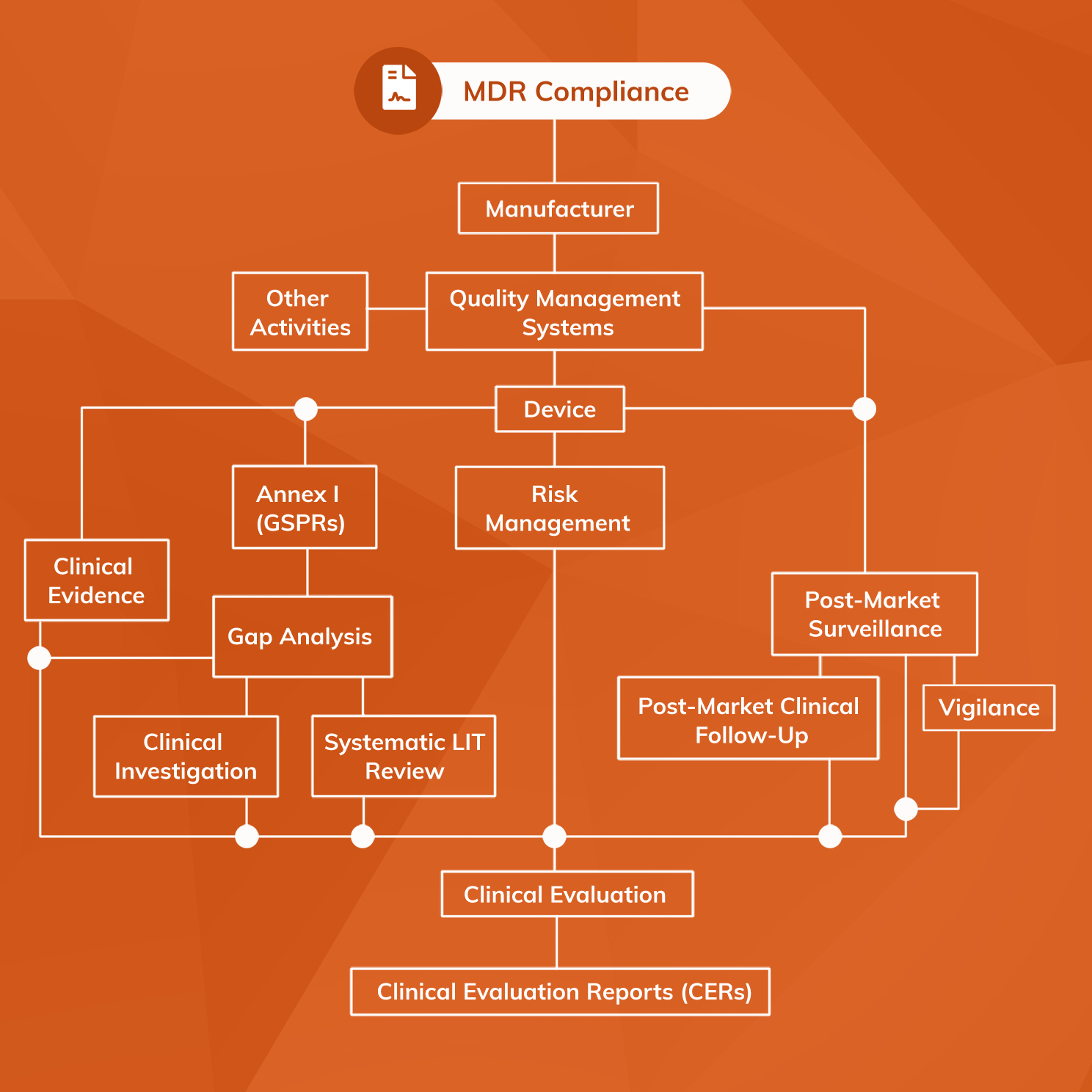
MDCG Guidance for Medical Device Software | Freyr - Global Regulatory Solutions and Services Company
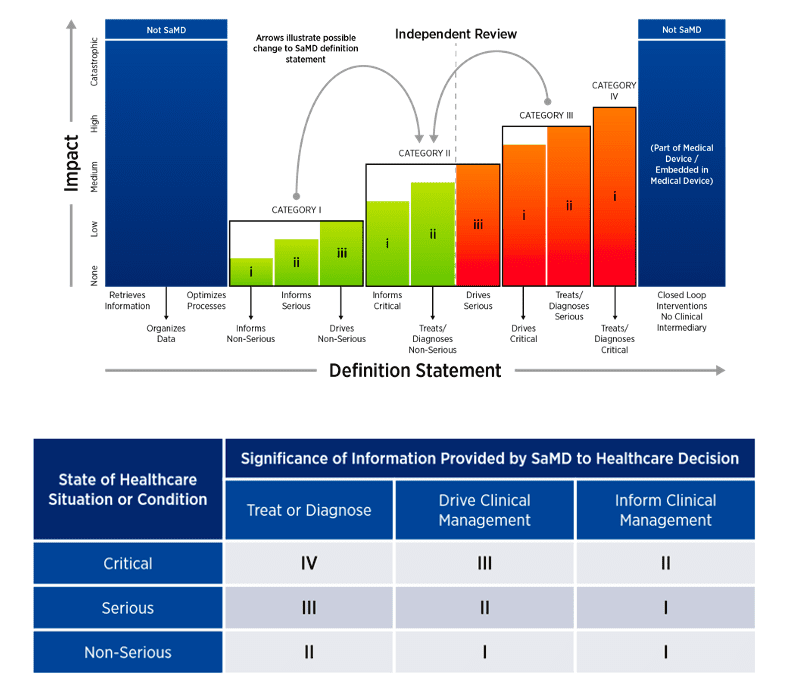
Software as a medical device: Here's how the regulatory landscape is changing - Medical Design and Outsourcing